Insights
Green Ammonia: new prospects and challenges ahead
7 August 2025
As the essential building block for all mineral nitrogen (N₂) fertilisers, ammonia (NH₃) transforms inert N₂ from the atmosphere into a form that plants can use, turning an inert gas into agricultural harvests. Currently, around 70% of ammonia is used to produce fertilisers, while the remainder is consumed as an essential feedstock in chemical processes to make plastics, explosives, and synthetic fibres.
Since its large-scale adoption in the early 20th century, the Haber-Bosch (H-B) process has been the dominant method for commercial ammonia production. The major drawback of the conventional H-B process is its high energy requirement, in the region of 40 GJ per tonne of ammonia produced (Figure 1). This is due to the extremely thermodynamically stable N-N triple bond, resulting in high temperatures (350-500°C) and high pressures (150-300 bar) [1]. Ammonia production through the H-B process accounts for approximately 2% (8.6 EJ) of total global energy consumption - its heavy reliance on fossil fuels makes it a major contributor to CO₂ emissions, accounting for 5% of total industrial CO₂ emissions [2].
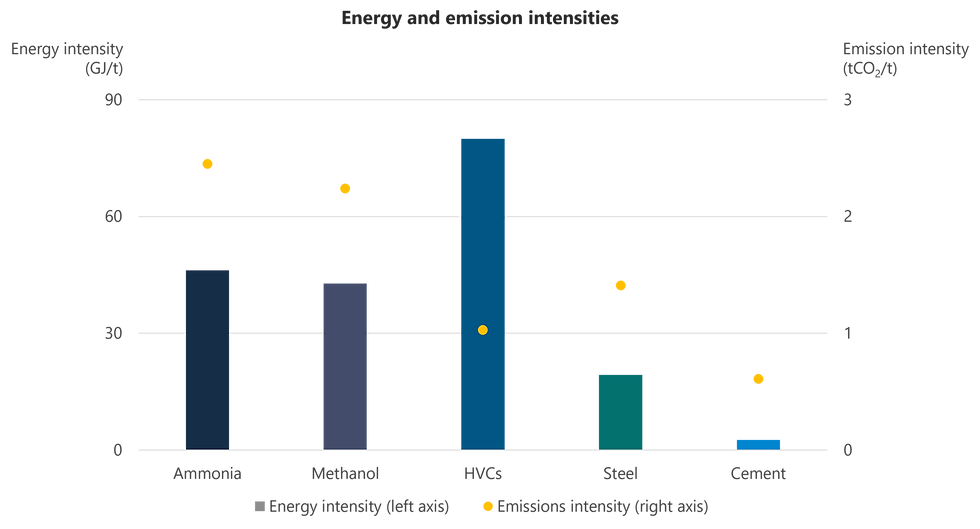
Figure 1: Energy consumption for and emissions from ammonia production, 2020 [2]
(HVCs = high value chemicals, including ethylene, propylene, benzene, toluene and mixed xylenes)
Emerging ammonia synthesis pathways
Under the decarbonisation backdrop, green ammonia synthesis technologies have gained significant commercial and research interest, especially the green H-B process, which uses renewable electricity for H₂ generation as well as the ammonia synthesis reaction. This promising process has attracted numerous commercial players, including startups such as TalusAG, Nium, First Ammonia, and Ammobia. Although the green H-B approach is regarded as promising for commercial-scale production, it faces challenges, including the need for a large quantity of high-purity water for H₂ generation [3]. In terms of nitrogen generation, the air separation unit (ASU) requires a substantial amount of electricity, which continues to make the green H-B process energy-intensive [4].
In addition to green H-B, our recent research has identified 14 alternative approaches for producing green ammonia (Table 1), all of which remain largely at the laboratory stage within academic and research institutes, with low technology readiness levels (TRLs). However, five pathways (Figure 2) have attracted commercial interest.
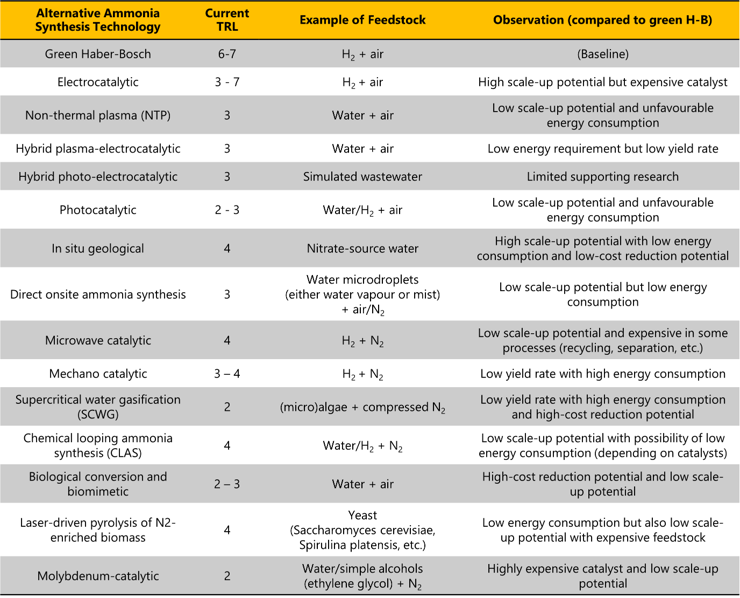
Table 1: Alternative ammonia synthesis routes, current TRLs and key observations (compared to green H-B)
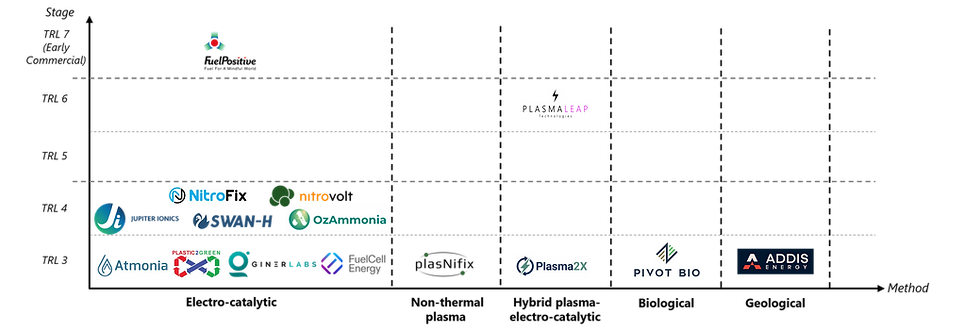
Figure 2: Emerging players for alternative green ammonia synthesis (non-green H-B), including FuelPositive, NitroFix, NitroVolt, Jupiter Ionics, Swan-H, OzAmmonia, Atmonia, Plastic 2 Green, Giner Labs, FuelCell Energy, plasNifix, PlasmaLeap, Plasma2X, Pivot Bio, and Addis Energy
The electrocatalytic pathway: high research interest and intensity
Following the green H-B process, the electrocatalytic approach is currently the second most technologically mature method for green ammonia production. This process typically operates under ambient temperature and pressure, using only air and water as feedstocks.
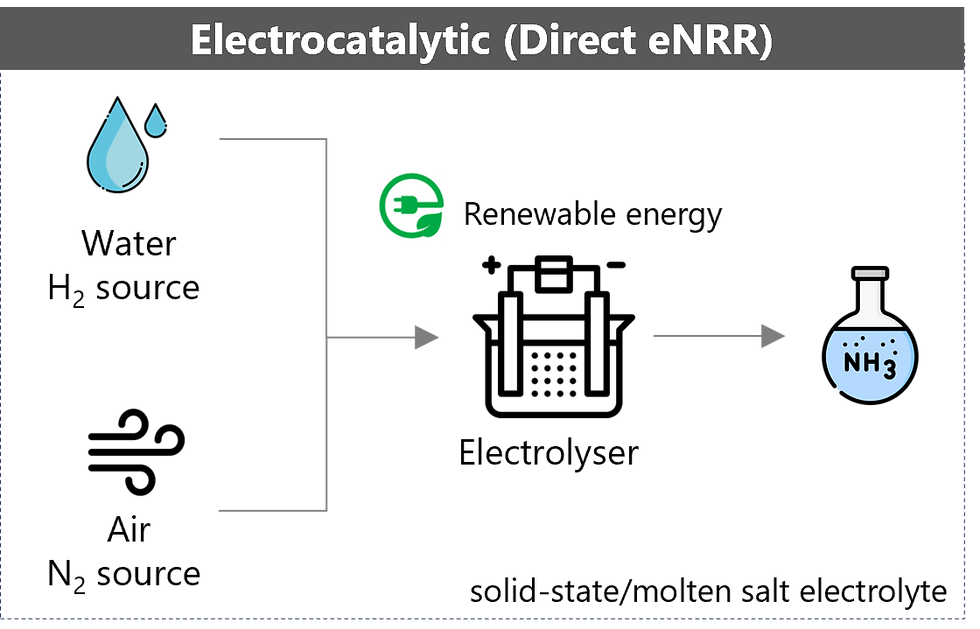
This offers considerable energy-saving potential, assuming scalability to the commercial production stage. Additionally, the process can be coupled directly with renewable electricity sources, enabling smaller-scale and decentralised production. Several commercial companies are investing in the development of the electrocatalytic process. FuelPositive, a Canadian company established in 2020, has made good progress toward deploying the first commercial system for on-farm ammonia production, using only air, water and renewable electricity.
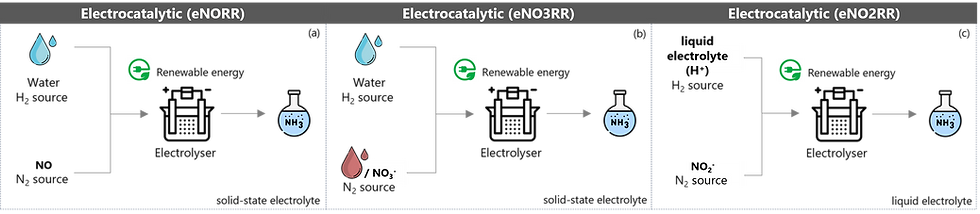
Figure 3: Electrocatalytic green ammonia synthesis, including (a) electrochemical nitrogen reduction reaction (eNORR), (b) electrochemical nitrate reduction reaction (eNO3RR), (c) electrochemical nitrite reduction reaction (eNO2RR)
One of the benefits of the electrocatalytic technology is the variations of feedstocks and electrolytes. Figure 3 highlights multiple subcategories within the electrocatalytic route that employ NOₓ species as feedstocks, offering several advantages, particularly in achieving higher NH₃ yields compared to the eNRR approach (using air directly).
Weaker bond of N-O: the N-O bond is weaker and less stable than the triple bond of N₂, so breaking it in the eNOₓRR process requires less energy, making the reaction thermodynamically more favourable.
Greater water solubility to N₂: NOx is a polar and reactive gas that can interact with or dissolve by reacting with water, while N₂ is nonpolar and chemically inert.
Cost reduction by eliminating the ASP unit: The eNOₓRR process does not require an ASP unit, making it more cost-effective.
Reducing air/water pollution: The eNOₓRR process offers the added benefit of mitigating air and water pollution through NOₓ removal as it prevents the formation of acid rain and nitrogen-related water contaminants.
In terms of the electrolyte, although a liquid electrolyte enables faster reaction rates, its environment can promote the reaction of H⁺ ions with each other to form H₂ rather than reacting with N₂. In contrast, a solid-state electrolyte helps suppress unwanted H₂ formation.
However, the major drawback of the electrocatalytic approach is its much lower ammonia yield rate compared to the H-B process - currently insufficient to meet commercial demands. In addition, the high production cost, mainly due to the use of expensive catalysts, further limits its practicality. Moreover, most research is still confined to the laboratory, primarily exploring new catalysts and alternative electrolytes. Therefore, the timeline for commercial-scale implementation remains uncertain.
The hybrid plasma–electrocatalytic ammonia synthesis: high commercial promises
The hybrid plasma–electrocatalytic ammonia synthesis approach combines non-thermal plasma (NTP) and electro-catalysis, with the N₂ species first undergoing plasma activation to form NOx or activated nitrogen species (N₂*), which are nitrogen molecules energised into an excited state, making them much more reactive in chemical reactions. NOx or N₂* are then electrocatalytically reduced into NH₃. The formation of such reactive intermediates significantly helps to overcome the inherent inertness of the N₂ molecule.
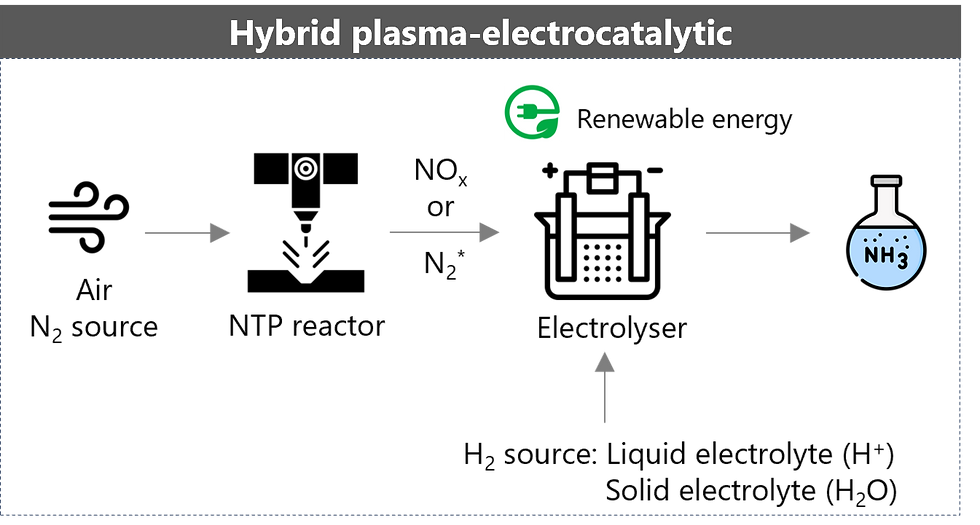
NTP provides the necessary energy to break the strong N₂ triple bond through electron-impact reactions, thereby creating highly reactive species that can then participate in the formation of ammonia under significantly milder conditions. Additionally, NTP offers potential for the use of more abundant and cost-effective catalyst materials, such as transition metal oxides. Currently, various types of plasma reactors are under development to address key challenges such as low energy efficiency, complex designs, and high energy consumption at the laboratory scale. The Swiss startup plasNifix is advancing plasma technology that employs gaseous catalysts at lower temperatures; however, its process still appears to be at the laboratory stage.
The hybrid approach seeks to leverage the strengths of both technologies to improve the overall production rate and is theoretically more energy efficient, and could enable decentralised production. Although significant challenges still remain, such as low yield rate and low scale-up potential, PlasmaLeap has been developing reactors using air, water and renewable electricity through this method. This University of Sydney spin-off has advanced to the demonstration stage in real farm settings (Figure 4).

Figure 4: the ammonia production unit from PlasmaLeap [5]
In situ geological ammonia synthesis: high scale-up potential
An interesting development from last year came from the MIT spin-off Addis Energy, which introduced an innovative in-situ geological method for synthesising ammonia (AKA Geo-NH₃). The process (Figure 5) involves injecting nitrate-rich water into iron-rich geological formations, where the reaction occurs underground. This approach utilises an extremely simple feedstock, requiring neither H₂ input nor additional energy or electricity. Copper ions (Cu²⁺) are the most effective catalysts for geological NH₃ synthesis, followed by nickel (Ni²⁺) and manganese (Mn²⁺) ions. These catalysts can either be introduced into the aqueous solutions injected underground to enhance the rate of Geo-NH₃ production or occur naturally in certain rock formations, as identified in regions such as Costa Rica, Oregon, and California [6].
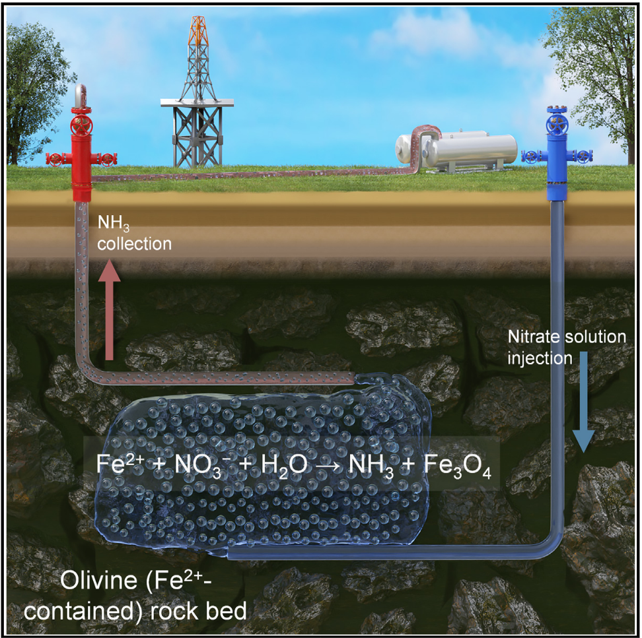
Figure 5: schematics of the in-situ geological ammonia synthesis process [6]
Compared to other pathways, this process offers a significantly more straightforward scale-up potential by leveraging the significant know-how from the oil and gas industry and the geothermal industry. Our analysis suggests that this approach might be cost-competitive:
CAPEX primarily includes expenses related to borehole drilling and rock fracturing
OPEX covers ongoing costs such as nitrate sourcing, water and catalyst supply, logistics, and related operational activities
The cost of NH3 is estimated to be around $0.55 per kg of NH₃ when using NO₃⁻ as the nitrogen source. This cost could drop to $0.46 per kg NH₃ if the geological NH₃ synthesis is performed on ultramafic rock formations naturally containing Ni and Cu catalysts. The use of nitrogen sources combined with naturally occurring catalysts brings the cost of Geo-NH₃ close to parity with that of conventional grey ammonia produced via the H–B process ($0.4 per kg NH₃).
Potential Exists, but there is a Long Journey to Commercial Breakthrough
While a few commercial players are beginning to emerge, the majority of alternative green ammonia synthesis approaches remain confined to academic research with limited real-world applications to date. To unlock the full potential of green ammonia synthesis, several pressing challenges must be tackled.
Feedstock availability and cost
The production of H₂ and N₂ remains a significant cost barrier. As highlighted in our previous article, green H2 is expected to remain prohibitively expensive for at least the next decade. Additionally, large-scale N₂ production typically relies on energy- and capital-intensive ASUs. To address these challenges, innovative approaches are being explored, including geological H₂, solid oxide electrolysis cells (SOEC) for integrated ammonia feedstock production, supercritical water gasification (SCWG) of biomass for H₂ generation, and chemical looping air separation for more efficient N₂ production.
For instance, geological H₂, also known as “white H₂” or “orange H₂”, is naturally generated through a chemical redox process called “serpentinization”, in which iron-rich ultramafic rocks undergo oxidation while simultaneously reducing underground water to produce H₂. Currently, major energy players such as Shell, BP, and Chevron have joined a consortium founded by the U.S. Geological Survey and the Colorado School of Mines to investigate natural, or geological H₂ [7]. At the same time, a number of innovative start-ups are actively pursuing the exploration and development of this promising resource. Although the costs associated with drilling injection wells and subsurface analysis can be challenging, these limitations might be overcome by economies of scale.
Engineering scale-up
All pathways face substantial engineering scaling-up challenges. Maintaining energy efficiency, reaction stability, and overall system performance at industrial scales remains a key hurdle in their development and deployment. For example, scaling up plasma reactors faces key challenges due to the complex interplay of plasma parameters, reactor geometry, maintaining consistent plasma characteristics and ensuring uniform treatment across larger volumes. Even at the laboratory scale, the underlying reaction mechanisms are highly complex and not yet well understood, especially in terms of plasma physics, gas-phase chemistry, and interactions between surfaces and catalysts. As noted earlier, catalysts for plasma technology are relatively cost-effective; however, knowledge of their stability under plasma conditions is still limited, since factors such as erosion and poisoning have not been extensively studied. Moreover, energy losses in the form of heat, UV/visible light, and non-productive collisions have been observed, thereby reducing the overall process efficiency [8]. Several universities and research institutions are actively working to develop plasma technology for ammonia synthesis; however, it remains far from achieving commercial-scale application in the near term.
Production and yield rates
Many emerging approaches report low and inconsistent production/yield rates under a lab environment, limiting their practical viability and making it difficult to benchmark or compare performance to the H-B process. Even the most promising approach, the electrochemical process (Figure 6), achieves an overall yield that is still relatively low (larger yield rate up to 1 mmol cm−2 h−1) compared to the H-B process, at about 12% conversion [9]. During our research, we see that no consistent or repeatable results have yet demonstrated a particular setup that reliably delivers high yields. Other approaches, such as the photocatalytic process, face the same issue, as experiments are often conducted using different methods or under varying conditions. The resulting data are frequently reported in different units, which creates another difficulty for comparison. Altogether, these issues hinder reproducibility and make the objective evaluation and comparison of KPIs extremely challenging.
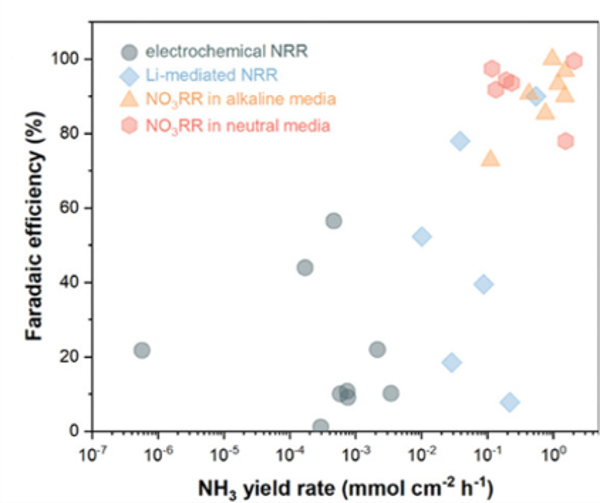
Figure 6: Faradaic efficiency vs. NH₃ yield rate for eNRR, Li-NRR and NO3RR (alkaline and acidic media) [9]
Separation and recycling
In alternative ammonia production approaches, the separation and recycling of ammonia remains significantly underexplored. This presents a major barrier to commercial viability, as the lack of efficient and scalable recycling methods hinders the overall process efficiency. This research [10] on NTP ammonia synthesis indicates that the inherently low NH₃ production rates lead to high energy demands for both reactant recycling and product separation due to:
Extremely low conversion yield: requiring continuous recycling of unreacted feedstock, leading to high energy consumption and reduced process efficiency.
Large recycle compressor requirements: requiring a high-capacity recycle compressor, which is expensive and energy-intensive
Low NH₃ partial pressure: causing the condensation of NH₃ separation to be ineffective and requiring solid sorbents like metal halides or zeolites, which are costly and bulky.
To achieve commercial viability comparable to the H–B process, the total energy consumption should be targeted at approximately 80 GJ per tonne of NH₃ (Figure 7). However, in the NTP method, the combined energy demand for NH₃ separation using sorbents and reactant recycling alone exceeds 100 GJ per tonne of NH₃, excluding the additional energy required for gas purification and ammonia synthesis [10].
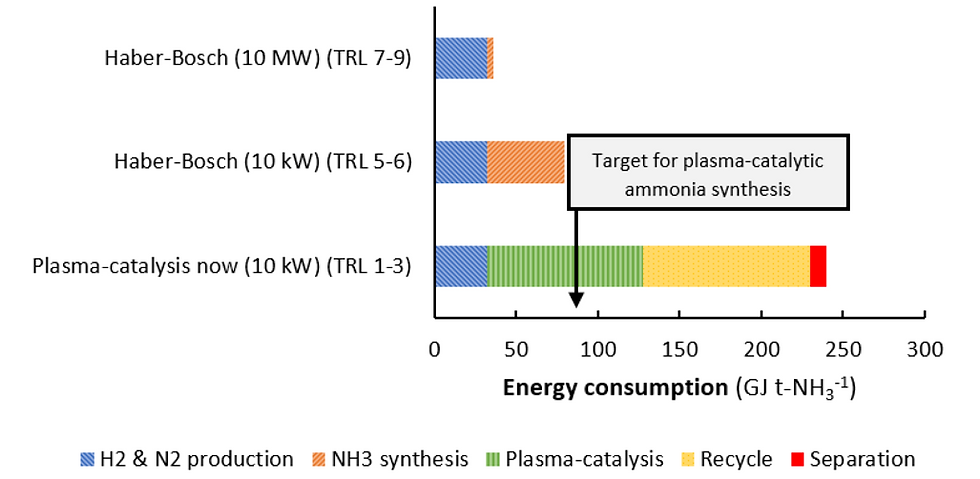
Figure 7: Estimated energy consumption of ammonia synthesis: Large-scale electrolysis-based H-B, small-scale electrolysis-based H-B, and plasma catalysis [10]
For ammonia synthesis, the total energy efficiency and conversion rate are the primary KPIs. Unfortunately, all alternative approaches that we examined are far from achieving commercial viability in the short-term frame.
References:
[1] Ammonia: zero-carbon fertiliser, fuel and energy store : policy briefing. London: Royal Society; 2020.
[2] International Energy Agency. Ammonia Technology Roadmap: Towards more sustainable nitrogen fertiliser production. OECD; 2021. Available from: https://www.oecd.org/en/publications/ammonia-technology-roadmap_f6daa4a0-en.html
[3] Ghavam S, Vahdati M, Wilson IAG, Styring P. Sustainable Ammonia Production Processes. Front Energy Res. 2021 Mar 29;9:580808.
[4] Yan R, Wu M, Fan J, Sun C, Wang J, He Y, et al. Process integration and thermodynamic analysis of a multi-generation system including solar-assisted biomass gasification and chemical looping ammonia generation. Energy Convers Manag. 2024 Apr 15;306:118263.
[5] PlasmaLeap: eNFix - Green, Competitive Nitrogen fertilisers, on-farm. Available from: https://www.plasmaleap.com/enfix
[6] Gao Y, Lei M, Kumar BS, Smith HB, Han SH, Sangabattula L, et al. Geological ammonia: Stimulated NH3 production from rocks. Joule. 2025 Jan 21;0(0). Available from: https://www.cell.com/joule/abstract/S2542-4351(24)00541-5
[7] Aliyu A. Gold, Geologic, White, Native, Hidden, Natural Hydrogen: Does Earth hold extensive stores of untapped, carbon-free fuel? IEAGHG. 2024. Available from: https://ieaghg.org/insights/gold-geologic-white-native-hidden-natural-hydrogen-does-earth-hold-extensive-stores-of-untapped-carbon-free-fuel/
[8] Plasma-Conversions-public-report.pdf. Available from: https://ispt.eu/media/Plasma-Conversions-public-report.pdf
[9] Xiong Y, Wang Y, Zhou J, Liu F, Hao F, Fan Z. Electrochemical Nitrate Reduction: Ammonia Synthesis and the Beyond. Adv Mater. 2024;36(17):2304021.
[10] Rouwenhorst KHR, Lefferts L. Feasibility Study of Plasma-Catalytic Ammonia Synthesis for Energy Storage Applications. Catalysts. 2020 Sept;10(9):999.